The textile manufacturing industry encompasses many and diverse processes that rely heavily on the use of water, energy, chemicals, and other resources. Wet spinning, sizing, desizing, scouring, bleaching, mercerization, dyeing and printing are just a few. Monitoring and controlling the pH, TDS/Conductivity/Salt Concentration, ORP (REDOX), and Temperature of the aqueous solutions used in these processes conserves costly resources, controls quality, and reduces the amount of pollution that must be treated before discharge of effluent wastes. This can be done manually with handheld instruments or automatically with in-line monitor/controllers.pH
In all textile processes in which aqueous solutions are used, balancing the pH of the solution is primary. pH control is critical for a number of reasons. The effectiveness of oxidizing and reducing agents is pH dependent. The amount of chemicals required for a given process is directly related to the pH. The solubility of substances, such as dyes and impurities, vary with pH. The corrosive and scaling potential of processing solutions is also heavily influenced by pH. All of these issues affect quality and costs. Along with surface tension, pH plays an important role in the wetting and saturating processes. For example, caustic solutions cause interfibrillar swelling in cotton cellulose and cannot be squeezed out as easily as water, which can reduce quality in subsequent processing. The scouring of wool is a good example of a process where maintaining the pH value permits a better solubilization of certain impurities. For example, a pH of 8.5 is considered optimum for the removal of wool wax.
In the instance of vat dyeing, pH controls the solubilization of the dyes. Initially, the quantity of caustic soda present must be adequate to ensure the solubility of the leuco form. Once the dye has been exhausted, the pH is adjusted such that the dye returns to its insoluble form and is mechanically trapped in the fiber. Between the color kitchen and processing, controlling the pH improves the lab-to-bulk reproducibility of color. Monitoring and controlling pH ensures consistency of color from batch to batch, as well.
Maintaining the correct pH is also critical in processes where a specific pH permits a reaction mechanism necessary for the purification of fibers: bleaching with sodium hypochlorite or hydrogen peroxide, desizing with oxidizing agents or removing soluble products and others. To effectively bleach cellulose (e.g. cotton) with a minimum amount of damage, the bleaching solution must be alkaline. This keeps the hypochlorite stable and also prevents the presence of reducing groups that cause an apparently well-bleached cloth to yellow with age. Additionally, an acidic solution will form toxic and corrosive chlorine gas. Bleaching liquor is therefore usually maintained at a pH of 9. The permanence of the white obtained is thereby increased, and the bleaching is safe.
Due to environmental concerns in recent times, hydrogen peroxide bleaching has become more prevalent. Its reaction products, oxygen and water, are relatively harmless. However, hydrogen peroxide is a weak acid. Thus its conjugate base, HO2-, is used to perform the actual bleaching. To ensure an adequate concentration of HO2 -, the solution pH must be tightly controlled. Sodium hydroxide is used to maintain the pH at a very alkaline level of 12-12.5. pH must also be maintained in desizing, where hydrogen peroxide, sodium bromite, or other oxidizing agents are employed to remove the size of the yarn. A pH of >9 is required in the use of sodium bromite, for example, as it decomposes at pH 8. The pH of the boiler water in textile processing must also be carefully monitored and controlled. To minimize corrosion, a pH as high as 9 is desirable.
However, care must be taken to avoid excess alkalinity. Alkaline substances such as sodium hydroxide, carbonate, and phosphate have a solvent action on the non-ferrous metal fittings. In pressure boilers caustic embrittlement may also occur at riveted areas and places of high stress. Additionally, it must be considered that acidic waters cannot be softened by agents such as EDTA to improve processes.
Myron L Company manufactures a variety of handheld instruments and in-line monitor/controllers that measure and manage pH. Use the digital handheld Ultrameter II™ 6P or TechPro II™ TH1 to measure the pH of solution. Use Myron L in-line pH monitor/controllers such as the 720 Series II to establish and maintain an acceptable process solution pH setpoint automatically.
——————————————————————————————————————–
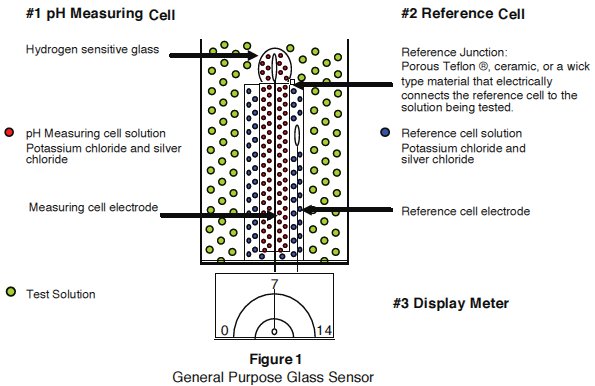 Figure 1 : General Purpose Glass Sensor
Figure 1 : General Purpose Glass Sensor
What is pH? : pH is the negative logarithm of hydrogen ion activity in a solution. The Concentration ratio of hydrogen ions (H+) and hydroxyl ions (OH-) determine the pH value of a solution. Any hydrogen activity will produce a 59.16 mV/pH unit across the glass membrane. The measurement is expressed on a scale of 0.0 to 14.0. Water with a pH of 7 is considered neutral (H+ ions = 10-7 and OH- ions =10-7). A solution is considered acidic when the hydrogen ions (H+) exceed the hydroxyl ions (OH-), and a solution is considered an alkaline (base) when the hydroxyl ions (OH-) exceed Hydrogen ions (H+).
How is pH measured? : A pH instrument consists of three main components, refer to Figure 1 above.
The pH measuring cell: Hydrogen sensitive glass is blown onto the end of an inert glass stem. A silver wire, treated with silver chloride (Ag/AgCl) is sealed inside the glass (cell) with a solution of potassium chloride saturated with Silver chloride. The measuring solution has a neutral pH level of 7 or 0 mV. A properly hydrated glass sensor will produce a “Gel Layer” on the inside and outside of the glass membrane. The “Gel Layer” enables hydrogen ions to develop an electrical potential across the pH glass sensor; a millivolt signal varies with hydrogen ion activity on the glass membrane while submerged in the solution being tested.
The Reference cell: A silver wire treated with silver chloride (Ag/AgCl) is sealed inside an inert glass housing (cell) with a solution of potassium chloride saturated with silver chloride. The inert glass prevents hydrogen ion activity from test solutions to influence the reference cells constant millivolt signal. The combination of the reference electrode silversilver chloride wire, and the saturated potassium chloride solution develops a constant 199-millivolt reference signal. The millivolt signal produced inside the reference electrode does not vary as long as the chloride concentration remains constant. The reference voltage is used as a baseline to compare variations or changes in the solution being tested. The reference cell is in contact with the test solution through a reference junction that is commonly made of porous Teflon®*‚ ceramic, or a wick type material called a Pelon strip. This junction completes the measuring circuit of the pH sensor.
Display meter: When the pH sensor is placed in a solution, the pH-measuring cell develops a millivolt signal that reflects the hydrogen ion activity of the test solution. A high impedance meter accurately measures the small millivolt changes and displays the results in pH units on either an analog meter or digital display.
——————————————————————————————————————
Total Dissolved Solids (TDs) and Conductivity
The concentration of dissolved solids in solution, such as metal salts, is determined by a measure of the conductivity of a solution and may be converted to parts per million total dissolved solids. TDS/Conductivity affects quality and energy efficiency in many processes from the boiler water to the dye bath. A TDS reading of 65-150 ppm is generally considered suitable for the textile industry. If the source water has too high a TDS, it can significantly increase energy costs and result in a poor quality product. In the case of boiler water, impurities reduce efficiency of the boilers and can lead to corrosion of equipment. Scale also forms, which is a poor conductor of heat. In addition to increased fuel consumption then required, the presence of scale is dangerous as it causes overheating, which softens the metal and ultimately causes it to fail. TDS is also a good way to measure precise concentrations of salts used in the exhaustion of direct dyes on cellulose fibers, such as cotton. It is important to determine the optimum level of salt as there is a maximum beyond which further additions may not yield much effect. Using this optimum level conserves salt use and ensures the most rapid exhaustion of vat dyes. Measuring TDS of rinse water in the vat dye process is useful in determining the effectiveness of the rinsing. By taking a reading of rinse water from an ideal product and measuring it against readings from batch to batch, you can determine when the product has been properly rinsed. This ensures product quality while conserving water. Monitoring and controlling TDS in the color kitchen and in the dyeing process itself greatly improves color reproducibility from the kitchen to the batch. Measuring and controlling TDS in the dye bath from batch to batch also ensures quality consistency.
Salt concentration is also an important issue to be managed in dyeing process wastewater, especially where reactive dyes are used. Cotton batch dyeing operations typically use quantities of salt ranging from 20 to 80 percent of the weight of the fabrics. High TDS in effluent can also indicate the presence of inorganic pollutants. Dyeing effluents, for example, can contain concentrations of some heavy metals such as chromium and copper, but also zinc, lead, and nickel.
Use the handheld Ultrameter II 4P or 6P, TechPro II TP1 or TPH1, or DS/pDS meter to manually test TDS/Conductivity and Salt Concentration, cooling the water to the prescribed parameters for the instrument, if necessary. To automatically monitor and control TDS/Conductivity or Salt Concentration in any solution, use a 750 Series II Monitor/controller.
Oxidation Reduction Potential (ORP)
ORP is the best way to measure the true oxidizing or reducing power of all chemicals in solution. It is, therefore, an excellent way to measure and control quantities of sodium hydrosulphite, for example, and other sulfur reducing agents and bleaches. This drastically reduces the amount of error in determining chemical quantities required in traditional dyeing processes, for example. Precisely controlling the quantity of the reducing agent keeps the concentration to a minimum and provides that there is enough to avoid oxidation of the dye. It also prevents overreduction of dyes, which results in poor color yields. During continuous reduction washing of disperse dyed or printed materials, maintaining the proper ORP ensures the residual dyes are cleaned from solution and have no chance of migrating and reattaching in undesirable areas of the fabric. ORP measurement should be taken periodically during printing to ensure an adequate quantity of reducing agent is present throughout the entire process. In all of these processes, monitoring and controlling ORP conserves chemicals and costs while reducing pollutants that must be treated in wastewater before discharge. Another important benefit is a substantial decrease in the noxious fumes present in the factory.
Use the Ultrameter II 6P to manually monitor and control chemical levels using ORP and as a quick check against automatic systems of control. Measurements can be taken of automated system samples or in open-width washers, which is necessary for printed fabrics. Use the 720 Series II pH/ORP Monitor/controller to establish a setpoint and automatically open and close the valve to the oxidizing/reducing agent to maintain the correct chemical levels.
Temperature
Solution Temperature directly impacts the rate of processes and the solubility and availability of chemicals and thus the quality of the product. For example, it is important to monitor and maintain the dyebath such that maximum exhaustion is obtained and dye uptake optimized. For normal dyeing times, as temperature increases, the amount of dye on the fabric increases up to a point. This point is called the temperature of maximum affinity, and it may differ from the temperature at which there is the greatest concentration of dye in solution. Increasing the temperature beyond this point actually causes loss of dye from the fabric. Lower temperatures may cause the dyeing process to take longer. So to ensure quality and conserve energy, the solution temperature must be carefully monitored and controlled.
The Ultrameter II 4P, 6P and the TechPro II, TPH1, TP1 and TH1 all conveniently measure temperature with each sample taken. Both 720 & 750 Series II have temperature measurement/control as an option.
Waste Water Management
The Ultrameter II can be used as a completely portable method of monitoring up to 6 basic parameters in wastewater: Conductivity; TDS; Resistivity; ORP (REDOX); pH; and Temperature. The Ultrameter II can store up to 100 date-time-stamped readings in memory. You can instantly download stored data to any computer using the optional uDock™ accessory package for easy data entry, analysis, and reporting.
Founded in 1957, the Myron L Company is one of the world’s leading manufacturers of water quality instruments. To find out more about how Myron L water quality instrumentation, please contact :
Myron L
2450 Impala Drive, Carlsbad, CA 92010-7226 USA Tel: +1-760-438-2021 Fax: +1-800-869-7668 (www.myronl.com)





