Nanotechnology is currently being used as a valuable weapon for combating body odor-causing bacteria. Materials can be manufactured at the ‘nano’ scale, one billion times smaller than the world of meters we currently live in. Nanoparticles provide terrific driving forces for diffusion, which allow s chemical reactions to occur at a high rate. In the case of odor-resistant clothing, the textile industry recognizes that silver nanoparticles offer distinct properties that combat bacterial growth and reduce body odor. Although nanoparticles can provide tremendous benefits, the potential effects on our health and environment still remains unknow n. Some of the primary concerns include the possibilities of nanoparticles penetrating skin and leaking into water systems. Thus, it is crucial for researchers to perform further studies in order to completely understand the impact of nanoparticles on our lives and the environment.
The term “nanoparticle” refers to any particle ranging from 1 to 100 nanometers in size, where a nanometer is one-billionth of a meter. To put the size of a nanoparticle in perspective, the diameter of a human hair is equivalent to 80,000 of the smallest nanoparticles combined. So what makes this miniature particle so popular in the scientific community? A single nanoparticle may not be not sufficient to make an impact, but a billion or even a trillion of these nanoparticles can be pow erful and beneficial to mankind. Scientists have worked with nanoparticles for centuries. Prior to the recent development of advanced microscopes, such as the Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM), scientists had faced significant limitations in their research due to the inability to see the structure of the nanoparticles. Today, the textile industry has integrated nanotechnology into novel products. Odor-resistant clothing, one of the current consumer products on the market, incorporates silver nanoparticles to help minimize the undesirable odor that results from bacteria in sweat and dirt (Fig. 1).
What’s That Smell?

Figure 1 : A Scanning Electron Microscopic (SEM) picture of silver nanoparticles loaded on grafted cotton fabric.
Most people believe body odor is an embarrassing, direct result of sweating or certain body traits. How ever, sweat itself is odorless. Body odors are actually caused by bacterial activity. Many microorganisms, such as bacteria, mold, mildew, and fungus, prefer to grow in moist environments, resulting in an unpleasant smell in our feet or armpits. Thus, bacteria thrive in our sweat glands. When bacteria grow on our body, they decompose sweat into acids that produce the odorous chemicals we perceive as body odor. Propionic acid and isovaleric acid are two common types of acids generated when bacteria break down the human body’s sweat. When amino acids are broken dow n by Propionibacteria into propionic acid, the acid produces a vinegar-like smell. Similarly, Isovaleric acid is produced when the bacteria species Staphylococcus epidermidis breaks dow n fatty acid, causing a cheese-like smell [1].
A Brief History of Silver and Nanoparticles
To minimize body odor, one must prevent the replication of microbes that are generated in our body sweat. Silver has a long history of being an antibacterial agent. Not only did ancient Greek and Roman civilizations use silver to disinfect water and food, but other pioneers also submerged silver coins in water and milk to keep the drinks fresh. By the 1920s, the U.S. Food and Drug Administration approved silver solution as a type of antibacterial agent. After the discovery of silver as an antibacterial agent, Richard Feyman, a physicist at Caltech, introduced the concept of the “nano-w orld” in 1959 in his famous lecture “There’s Plenty of Room at the Bottom.” Feynman suggested that in principle, it was possible to develop “nano-scale” machines capable of producing smaller products. Feynman suggested that if microscopic “machine shops” were created, then materials could be one billion times smaller than the current size to reach an unprecedented nano-scale level. Feynman proposed that smaller particles, with their low er mass, encounter less gravitational force, but experience greater influence by both Van Der Waals interactions and surface tension. His lecture on individual atoms and molecular manufacturing eventually stimulated scientists and engineers worldwide to develop technology to image, fabricate, and manipulate the fundamental structures of atoms and molecules [2].
Feynman’s proposition did not transform into a scientific concept until the 1980s and ’90s when Eric Drexler, along with other researchers, coined the term “nanotechnology”. Thereafter, engineers slow ly unwound the mystery inside the nanow orld Feynman had constructed in 1959. Along with increasing know ledge of the nano-w orld, scientists unveiled more unique features of nanoparticles. For instance, engineers noticed that, given their miniscule size, nanoparticles have a high surface-area-to-volume ratio, which means these particles also have a high reaction rate.
How Silver Nanoparticles Work on Clothing?
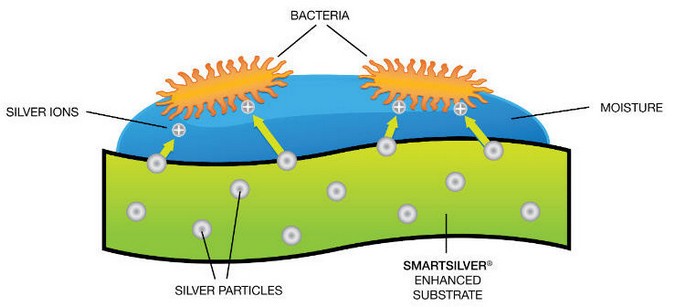
Know ing that silver is an effective antimicrobial agent and that nanoparticles provide terrific driving force for diffusion, how can we take advantage of these fantastic properties and integrate them into our everyday life to benefit us? From the antibacterial properties of silver and the applications of nanoparticles, engineers built “silver nanoparticles” to combine the major benefits offered by silver and nanoparticles together. Silver nanoparticles can be assembled into many different shapes, such as spheres, rods, cubes, wires, film, and coatings. Moreover, silver nanoparticles can be integrated into a variety of materials like metals, ceramics, polymers, glass, and textiles via fine spraying of silver nanoparticle solution [3]. Athletic clothing companies have incorporated silver nanoparticles into their products. Strolling dow n a sporting goods store, we can easily identify shoes, athletic clothing, and tow els labeled “antibacterial” or “odor controlling”, which usually refers to the use of silver nanoparticles in destroying odor-causing bacteria (Fig. 2) [4].
So how do these tiny particles actually destroy microbial colonies? Bacteria, approximately 1000 nanometers in size, use enzymes to metabolize nutrients and create energy in a similar fashion as any other living organisms. They are unicellular with only one compartment of protein, which stores all the elements of the cell. Thus, in order to stop the exponential rate of bacterial replication, it is necessary to disrupt the bacterial enzymes and energy metabolism.
To counter bacterial growth, the textile industry first inserts silver nanoparticles into its products to allow the particles to attach to the filaments. Once the silver nanoparticles encounter sweat from the human body or any other source of moisture, they naturally release a low concentration of silver ions into the moist environment. Unlike traditional antibiotics, which only concentrate on one of the pathways that kill bacteria, silver ions attack microbes in three different pathways: respiration, replication, and cell wall synthesis.

Trevira
Figure 3 : When bacteria takes up silver nanoparticles, its cell wall is damaged and the bacteria is killed.
Fig. 3 shows how silver nanoparticles penetrate the bacterial cell membrane and change their structural composition by interacting with the bacteria’s sulfate groups, which are the active site of enzymes. Silver ions disrupt the underlying means of bacterial survival by blocking some of the bacterial enzymes responsible for energy metabolism and electrolyte transport. The lack of enzyme activity ultimately suffocates the bacteria. As an additional means of attack, these powerful silver ions also deter the bacterial replication process by disrupting their DNA backbone.
Finally, silver ions bind to the bacterial cell wall to weaken the protection and structure of the cell, thereby creating structural imperfections within the cell’s protective layers and speeding the collapse or burst of the bacteria [5]. Therefore, by targeting these three areas, silver ions prevent bacteria proliferation by establishing a defense system, slow ing bacterial grow th, and eventually killing them.
Primary Concerns over Nanoparticles
Despite the useful properties in silver nanoparticles, there are underlying environmental and health concerns that must be addressed in order for the nanoparticles to comply with safety requirements. The Swiss Federal Laboratories for Materials Testing and Research conducted an experiment to examine the reduction of the amount of silver nanoparticles in fabrics subjected to washing. They discovered that silver nanoparticles were gradually stripped away from the fabrics in the washing machines and consequently discharged to the wastewater treatment plant [6, 7]. Since the silver material is highly toxic to microbes, these silver nanoparticles can reduce the efficiency of biological decomposition at the wastewater treatment plant. More importantly, they can also spread into the environment, polluting our soil and drinking water [8].
If the nanoparticles leak from treated wastewater or recycled water, they could pose a significant threat to the grow th of plants by killing beneficial soil microbes needed to keep the plants healthy. As a result, the nanoparticles can potentially damage crop production. In addition, if enough nanoparticles accumulate in the bio-solids at wastewater treatment plants, the fertilizers composed of bio-solids would also contain nanoparticles. Hence, the serial contamination of water and soil by nanoparticles could potentially yield adverse effects [9].
From household appliances to personal care products and medications, humans are exposed to increasing amounts of nanoparticles. According to scientific research, if industry continues to release mass amounts of nanoparticle-containing products, then the toxicity concentration from ions could negatively affect the aquatic ecosystem and potentially human beings as well. Gordon Shetler, an environmental news journalist, states, “In one new experiment at the University of Utah, Darin Furgeson, a professor of pharmaceutical sciences, exposed zebrafish embryos to silver nanoparticles in a laboratory, and found that some died and others were left with dramatic mutations [10].” Even though scientists have not confirmed the actual threat induced by nanoparticles on mankind, the concern revolves around the possibility of nanoparticles seeping into human skin and reaching into the organs. Once the nanoparticles enter the human anatomy, they can potentially leave us with long-term health effects. As a result, the EPA has enforced numerous regulations on the applications of nanotechnology in commercial industries to prevent ecological and health problems.
Future of Nanotechnology
Nanoparticle, a particle invisible to the human eyes, can be such a valuable weapon against microbial colonies and undesirable bacterial grow th. Yet, it could also infiltrate and defeat the immune system in the human body. Since nanotechnology is such a pow erful discovery that offers various applications, it is essential to understand all aspects of its impact on our ecosystem.
Existing methodologies can greatly benefit from process improvements designed to better evaluate the hazards of nanoparticles. We should never underestimate the influence of nanoparticles, since any misuse can lead to long-lasting health effects. “Nanotechnology is projected to be a trillion-dollar industry by 2015, with some saying it will be the focus of the next industrial revolution. The number of products – including sunscreens, paints, vitamins, food additives, electronics, vehicles and appliances – that use nano-materials has increased by nearly 380 percent since 2006, according to the Project on Emerging Nanotechnologies, a non-profit group based in Washington, D.C. that tracks nanotechnology [10].” Members of the science and engineering community are responsible for thoroughly understanding the pow er and implication of nanoparticles to allow us to safely integrate this promising technology into our daily lives.
References
- C. Nordqvist. What Is Body Odor (B.O.)? What Causes Body Odor? [Online]. Available: http://www.medicalnewstoday.com/articles/173478.php.
- L. Liu, “Background,” in Emerging Nanotechnology Pow er: Nanotechnology R&D and Business Trends in the Asia Pacific Rim, Singapore, World Scientific Publishing Co. Pte. Ltd., 2009 pp. 1-3.
- Dr R. Senje and I. Illuminat. (2009 June). NANO& BIOCIDAL SILVER[Online]. Available Telnet: http://www.foe.org/sites/default/files/Nano-silverReport_US.pdf
- E. Boysen and N. Boysen. Introduction to Nanotechnology [Online], Available : http://www.understandingnano.com/introduction.html.
- 2011. How SmartSilver Works[Online]. Available: http://www.smartsilver.com/how.
- (2010 Sept 1). Silver – and other – nanoparticles in fabrics[Online]. Available: http://oecotextiles.wordpress.com/category/chemicals/silver/
- L. Geranio, M. Heuberger, and B. Nowack. The Behavior of Silver Nanotextiles during Washing. Environmental Science & Technology 2009 43 (21), 8113-8118.
- N. Zeliadt. Silver Beware: Antimicrobial Nanoparticles in Soil May Harm Plant Life [Online]. Available: http://www.scientificamerican.com/article.cfmid=silver-beware-antimicrobial-nanoparticles-in-soil- may-harm-plant-life.
- E.S. Papazoglou and A. Parthasarathy, “Is Bionanotechnology a Panacea,” in BioNanotechnology, 1st ed. San Rafael:Morgan & Claypool, 2007 ch. 6, sec. 6.3.4, pp. 113-114.
- G. Shetler (2009 Nov. 17). Nanosilver in consumer products: No silverlining for fish [Online]. Available: http://www.environmentalhealthnews.org/ehs/news/nanosilver
(Courtesy : Illumin, the University of Southern California)